Ultrasonic Cleaning Solutions: What’s Inside and Why It Matters
Browse Volume:3 Classify:Support
When you drop a ring, a pair of eyeglasses, or a carburetor part into an ultrasonic cleaner, you might assume the device alone is doing the hard work. But while the high-frequency sound waves power the cleaning process, it’s the solution inside the tank that determines whether you’re getting mediocre results—or a deep, transformative clean.
Ultrasonic cleaning is built on a principle called cavitation. These microscopic shockwaves, triggered by ultrasonic frequencies traveling through a liquid, create tiny vapor bubbles that violently collapse. It’s this action that knocks grime loose from surfaces, even those buried in tight crevices. But cavitation needs the right kind of liquid medium to do its job well.
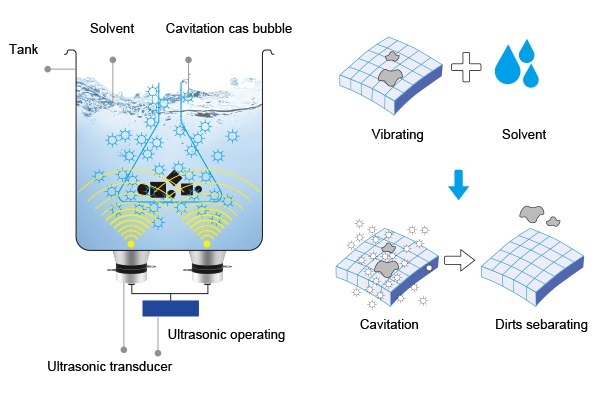
The Principle Behind Ultrasonic Cleaning
Pure water can conduct cavitation, but it lacks the chemical muscle to break down oils, carbon buildup, oxidation, and biological residue. That’s where a properly formulated ultrasonic cleaning solution comes in. It enhances the power of cavitation, penetrates grime, suspends debris, and protects delicate surfaces from recontamination.
The truth is, what’s in the tank matters just as much as what’s in your basket. Without the right formula, your ultrasonic cleaner is like a racecar with no fuel. That’s why understanding the ingredients, how they work, and how to choose the right solution is essential to getting professional-grade cleaning results.
What Exactly Is in an Ultrasonic Cleaner Solution?
The composition of ultrasonic cleaner solution varies depending on the application—jewelry, lab tools, automotive parts—but most formulas share a few common building blocks. Together, these ingredients not only help loosen and dissolve contaminants, but also protect the materials being cleaned.

Cleaning agent
Water as a Base—Why Distilled Water Is Best
Nearly all ultrasonic solutions begin with water, but not just any water. Distilled or deionized water is the preferred base because it contains no dissolved minerals, which could interfere with cleaning or leave behind residues. Tap water, especially in hard water areas, may contain calcium, magnesium, and iron that precipitate or react with cleaning agents.
Using distilled water ensures that the cavitation process is more efficient. It also helps extend the lifespan of both the cleaning machine and the item being cleaned. Some solutions come pre-mixed with water, while others are concentrates that need to be diluted—always with clean, mineral-free water.

Plain water
Surfactants and Their Dirt-Lifting Power
Surfactants are the chemical stars of ultrasonic cleaning. These molecules reduce the surface tension of water, making it easier for the liquid to penetrate cracks, pores, and microscopic recesses. More importantly, they lift away oily films and suspend particles in the liquid so they don’t settle back onto the object.
There are several classes of surfactants—anionic, cationic, non-ionic, and amphoteric—each with different effects:
- Anionic surfactants help emulsify oils and are strong against grease
- Non-ionic surfactants are milder and often used for delicate materials like lenses or jewelry
- Cationic surfactants sometimes serve a dual role as disinfectants
The right blend of surfactants ensures that the cavitation bubbles can get in, break debris loose, and keep the contaminants away from the surface afterward.
Chelating Agents That Break Down Minerals
If you’re cleaning metal parts, lab glassware, or any item exposed to water scale or oxidation, chelating agents are critical. These compounds latch onto metal ions like calcium, magnesium, and iron, neutralizing them before they can interfere with cleaning or cause staining.
One of the most common chelators is EDTA (ethylenediaminetetraacetic acid). It wraps around unwanted metal particles, making them easier to rinse away and preventing mineral re-deposition during the rinse cycle. In some acidic solutions, citric acid may serve a similar function.
Without chelators, you risk poor cleaning performance and the possibility of etching or pitting on soft metals or coatings.
Emulsifiers and Degreasers for Oils and Waxes
If your ultrasonic cleaning task involves removing lubricants, engine oil, cosmetics, or waxy buildup, the solution needs more than just surfactants. Emulsifiers and degreasers are added to help break oil into microscopic droplets that remain suspended in the solution and can be drained out with ease.
These ingredients are especially important in alkaline ultrasonic cleaning solutions, which target grease, carbon, and hydrocarbons. When properly formulated, they prevent redeposition of oil onto the item after cavitation.
Not all emulsifiers are safe for plastics or rubber components, so it’s important to choose a solution that matches the materials being cleaned.
Alkaline vs Acidic Ingredients and Their Targets
One of the most critical differences between ultrasonic cleaner solutions is whether the formula is alkaline, acidic, or neutral. Each pH level is suited to different cleaning tasks and materials:
- Alkaline solutions (pH 9–13): Best for grease, oil, carbon, and organic residues. Common ingredients include sodium carbonate or sodium metasilicate. Ideal for metals, ceramics, and glass—but not for aluminum or delicate jewelry.
- Acidic solutions (pH 2–5): Designed to remove oxides, rust, scale, and mineral deposits. Often contain citric or phosphoric acid. Suitable for descaling metal parts or removing corrosion, but can damage soft metals or plated surfaces.
- Neutral solutions (pH 6–8): Balanced formulas designed for general cleaning, especially where material sensitivity is a concern. Preferred for jewelry, optics, and electronic components.
Choosing the wrong pH can lead to surface damage, discoloration, or reduced cleaning performance. Always match the formula to the job.

Ultrasonic Cleaning Agents
Common Additives Found in Specialized Solutions
Beyond the foundational ingredients like surfactants and chelators, many ultrasonic cleaning solutions include additional agents tailored to specific applications. These additives enhance performance, protect the object being cleaned, and help the solution function reliably over time.
Let’s explore some of the most common—and crucial—specialty additives.
Rust Inhibitors for Metal Cleaning
When cleaning metal components, particularly iron or steel, there’s a risk of flash rusting once the part is removed from the solution and exposed to air. To prevent this, many solutions include rust inhibitors—chemicals that leave a protective film or modify the surface pH to deter oxidation.
These inhibitors are especially important in automotive, industrial, and firearm cleaning applications, where metal surfaces are vulnerable after washing. Some are designed to be rinsed off, while others are meant to remain as a temporary coating.
Without them, even freshly cleaned parts can start developing rust within minutes of exposure to air and humidity.
Enzymes for Biological Debris
In medical, dental, and lab environments, ultrasonic cleaners are often used to remove blood, tissue, and protein residues. For these applications, enzymatic cleaners are invaluable.
These solutions contain protease, lipase, and amylase enzymes that target different organic molecules:
- Proteases break down proteins
- Lipases break down fats and oils
- Amylases break down starches and carbohydrates
Because enzymes operate best at specific temperatures and pH levels, these solutions are often used warm (around 35–45°C) and within neutral pH ranges. Enzymatic solutions are non-corrosive and safe for delicate surgical instruments and labware.
Brighteners and Anti-Fogging Agents
In jewelry and optics cleaning, aesthetic clarity is critical. Some ultrasonic solutions include optical brighteners that help restore luster to metals and gemstones by reducing surface dullness.
For items like glasses, lenses, or camera filters, anti-fogging or anti-static agents may also be added. These reduce streaks, help prevent rapid condensation, and improve clarity after cleaning.
While not always essential, these enhancements elevate the final result, especially in display or commercial cleaning applications.
Foam Suppressants and Stabilizers
Foam is the enemy of cavitation. Excess bubbles in the liquid reduce the ultrasonic wave transmission and can even cause the machine to overheat or function improperly.
That’s why many professional solutions include anti-foaming agents, also called defoamers. These compounds reduce the surface tension of small bubbles, allowing them to burst and rejoin the solution more easily.
In concentrated formulas, stabilizers are also used to keep the active ingredients evenly mixed and effective over time, even during storage or dilution.
Different Types of Ultrasonic Cleaning Solutions and Their Uses
Not all ultrasonic cleaning solutions are interchangeable. Manufacturers formulate different blends depending on what you’re trying to clean—and what it’s made of. Choosing the right type ensures thorough cleaning without damaging the surface or reducing the effectiveness of the machine.
Here’s a closer look at the most common solution types and their intended uses.

Application
Neutral pH Solutions for General Cleaning
These are often labeled as “multi-purpose” or “safe for all materials”, and they’re a popular choice for first-time users or households. Neutral solutions are typically free of strong acids or bases and are safe for:
- Glass
- Plastics
- Most metals
- Precious stones
- Coated or painted surfaces
They may not remove baked-on grime or rust, but they provide consistent, safe cleaning with minimal risk of etching or discoloration. Ideal for jewelry, eyeglasses, watch bands, and tools.
Alkaline Solutions for Heavy-Duty Grease and Oil
These solutions have a pH above 9 and are designed to dissolve hydrocarbons, oils, carbon deposits, and industrial grime. They often contain detergents, emulsifiers, and mild solvents.
Alkaline solutions are widely used in:
- Automotive repair (engine parts, carburetors)
- Manufacturing (machined parts, dies)
- Aerospace (aircraft components)
- Firearms and gunsmithing
They should be used with care on aluminum, brass, and other soft metals, which can be damaged by prolonged exposure to high pH.
Acidic Solutions for Oxide and Scale Removal
When metal parts develop oxidation, scale, or mineral buildup, alkaline solutions won’t cut it. That’s where acidic cleaners come in. These are often based on citric, phosphoric, or sulfamic acid, and work best on:
- Iron and steel with rust
- Lab glassware with hard water scale
- Metal molds and fixtures with oxidation spots
Because they’re corrosive, acidic solutions require precise control of time, temperature, and dilution. They’re not suitable for coated, anodized, or plated surfaces unless specifically designed for those materials.
Enzymatic Cleaners for Medical or Dental Tools
Enzymatic solutions are the go-to choice for anything biological. They’re non-corrosive, biodegradable, and highly effective at breaking down:
- Blood
- Saliva
- Proteins
- Tissue residue
They’re used in hospitals, dental practices, veterinary clinics, and scientific labs—often as a pre-sterilization step. Because enzymes are sensitive to heat, most solutions work best around body temperature and must be handled gently to preserve activity.
Specialty Formulas for Jewelry, Electronics, and Optics
These are pre-formulated to avoid damaging fragile or sensitive materials. For instance:
- Jewelry cleaners may contain mild ammonia substitutes, brighteners, or anti-tarnish agents.
- Optics cleaners are alcohol-free and safe for anti-reflective coatings.
- Electronics-safe solutions avoid conductive or residue-forming agents and evaporate cleanly.
They often emphasize material compatibility and residue-free drying over brute cleaning strength. Perfect for ultrasonic cleaning of hearing aids, PCB boards, dental handpieces, and camera parts.
Are Homemade Ultrasonic Cleaner Solutions Safe?
It’s tempting to whip up your own ultrasonic cleaning solution, especially with so many online tutorials recommending everyday items like dish soap, vinegar, or baking soda. After all, if it works for scrubbing pots and pans, why not your jewelry or carburetor? But before you pour that homemade mix into your machine, it’s worth examining what really happens inside the tank—and whether your DIY brew is doing more harm than good.
Mixing Dish Soap and Water—When It Works
In simple cases, like cleaning lightly soiled eyeglasses or inexpensive jewelry, a diluted mix of mild, fragrance-free dish soap and distilled water can work reasonably well. Dish soap contains surfactants that reduce surface tension and lift light oils and dirt—similar to the surfactants found in commercial ultrasonic cleaning solutions.
However, dish soap is not optimized for cavitation. It may foam excessively, especially at higher concentrations, which disrupts the ultrasonic wave transmission. Excess foam creates a barrier that traps cavitation bubbles and insulates the surface from proper cleaning. That’s why even when dish soap works, it needs to be used very sparingly, and the solution must be closely watched for foaming.
What to Absolutely Avoid in DIY Solutions
Certain ingredients are simply incompatible with ultrasonic cleaning machines and should never be used:
- Bleach: Highly reactive, especially in warm solutions. It can corrode metal, damage plastics, and release harmful fumes.
- Hydrogen peroxide: Though popular in household cleaning, it breaks down under ultrasonic agitation and heat, potentially forming unstable byproducts.
- Ammonia: In strong concentrations, ammonia can damage coatings and corrode delicate parts. It’s also harsh on skin and eyes.
- Alcohols (isopropyl, ethanol): Flammable, and when used with heat or an open tank, pose a fire hazard.
- Acids (vinegar, lemon juice): May seem harmless, but can pit metal, loosen glued joints, and etch glass over time. Vinegar is especially corrosive in ultrasonic conditions.
These DIY solutions may provide a superficial clean, but they introduce significant safety and performance risks—both to your items and your machine.
Can Vinegar, Alcohol, or Ammonia Be Used?
The short answer: not directly. While some commercial ultrasonic solutions are formulated with diluted levels of ammonia or acetic acid (the key component in vinegar), they are chemically buffered, pH-balanced, and stabilized to prevent damage.
If you’re trying to remove tarnish from silverware, for example, it’s far better to use a commercial silver-safe ultrasonic solution than to guess at vinegar-to-water ratios.
Similarly, alcohol-based cleaners are used in electronics labs, but they’re applied in sealed, temperature-controlled ultrasonic units with proper fume extraction—not open tabletop units in homes or offices.
So while these ingredients may technically “work,” they’re not safe or recommended for use in standard ultrasonic cleaners.
Safety Concerns with Heat and Cavitation
Ultrasonic cleaning doesn’t just vibrate the liquid—it generates localized heat and high-frequency pressure zones. Many homemade cleaning mixtures break down under these conditions or become unstable.
For example:
- Homemade solutions can produce excess foam, which impairs cleaning and can overflow into sensitive electronic components.
- Non-neutral pH mixes (acidic or highly alkaline) can corrode the ultrasonic tank, especially if it’s uncoated steel.
- Unstable mixtures may emit vapors or splatter when agitated or heated, especially if the tank has a heating element.
If you’re using your ultrasonic cleaner regularly, especially on valuable or sensitive items, it’s best to invest in a properly formulated, purpose-built solution rather than risking damage with homemade concoctions.
How to Choose the Right Ultrasonic Cleaning Solution
With dozens of options on the market, choosing the right ultrasonic cleaning solution can feel overwhelming. But the decision becomes easier when you break it down by three simple criteria: what you’re cleaning, what it’s made of, and what kind of contamination you’re removing.
Matching Formula to the Contaminant Type
Your first consideration should be the type of grime or debris you’re trying to remove. Different residues require different chemical actions:
- Grease and oil? Alkaline solutions with strong surfactants and emulsifiers.
- Rust or scale? Acidic or descaling agents with chelators.
- Biological material? Enzymatic cleaners.
- General dust, fingerprints, or oxidation? Neutral solutions with mild surfactants.
Never assume that a single formula can handle everything—many solutions are optimized for a narrow use case, which is why professional shops often keep multiple solutions on hand.
Material Compatibility—What to Check First
Once you know what you’re cleaning off, you need to consider what you’re cleaning. Materials react differently under ultrasonic conditions, and the wrong formula can cause damage:
- Aluminum: Sensitive to high pH and acidic solutions.
- Plated metals: Risk of delamination if chemicals are too strong.
- Rubber or plastic components: May swell, soften, or degrade in harsh solutions.
- Jewelry stones (like opals or pearls): Require gentle, neutral-pH cleaners with no ammonia or alcohol.
- Optical lenses or coated glass: Need non-abrasive, residue-free, alcohol-free solutions.
If in doubt, check with the manufacturer or look for solutions labeled “safe for all materials” or “non-corrosive.”
Reading Product Labels for Active Ingredients
The best ultrasonic cleaning solution manufacturers provide transparent ingredient lists, usage instructions, and material compatibility tables. When choosing a product, look for:
- pH range
- Recommended dilution ratio
- Targeted applications (e.g., carburetors, surgical tools, jewelry)
- Any known restrictions (e.g., “do not use with soft metals”)
Avoid solutions that are vague, overly generic, or claim to clean “everything” with no specification. These are often diluted surfactants with minimal cleaning power.
When to Use Concentrates vs Ready-to-Use
Ultrasonic solutions come in two main formats:
- Concentrates must be diluted, usually at ratios of 1:10 or 1:20. These are more cost-effective over time and offer better control.
- Ready-to-use formulas are convenient but more expensive per use. Ideal for occasional users or small tabletop units.
If you clean items daily or operate a professional shop, concentrates are the smarter choice. Just be sure to use accurate measuring tools when diluting—guesswork can lead to ineffective cleaning or material damage.
How Ultrasonic Solution Ingredients Affect Cavitation
Cavitation is the heartbeat of ultrasonic cleaning. Without it, your machine is just a warm bath with a humming noise. But the cleaning solution you choose—and the chemicals inside it—directly influence how well cavitation works, how stable the process is, and whether your cleaning efforts succeed or fall flat.
Surface Tension: The Unsung Hero of Cavitation Efficiency
Cavitation works best when the liquid in the ultrasonic tank can form and collapse vapor bubbles rapidly and consistently. That requires a careful balance of surface tension—not too high, not too low.
Water has naturally high surface tension, which can hinder cavitation. Surfactants in cleaning solutions reduce that tension just enough to allow microscopic bubbles to form easily and burst with the right intensity. But if surface tension is lowered too much (say, by using too much detergent), the bubbles may become too soft, reducing the mechanical scrubbing effect.
In other words, effective cavitation relies on a solution with “just right” chemistry, allowing ultrasonic waves to generate powerful, uniform cavitation that reaches every crevice of the item being cleaned.
The Role of Viscosity and Temperature
A thicker solution—one with high viscosity—slows down the formation and collapse of bubbles. Most commercial ultrasonic cleaning solutions are formulated to maintain a water-like viscosity, even after being mixed or heated.
And yes, temperature matters. Cavitation is more intense when the liquid is warm—typically between 40°C and 60°C (104°F to 140°F), depending on the solution type. Many enzymes, surfactants, and chelating agents also perform better at these temperatures.
However, overheating a solution can degrade enzymes, destabilize emulsifiers, and even increase the risk of flash corrosion if rust inhibitors break down. This is why some ultrasonic cleaners come with temperature controls—so the cleaning chemistry stays within its optimal range.
Bubble Stability and Detergent Interference
Not all bubbles are helpful. Foam and soap bubbles differ from cavitation bubbles. When foaming agents are present—or when dish soap or shampoo is mistakenly added—these surface bubbles interfere with ultrasonic waves. They absorb energy, block signal transmission, and reduce the power reaching the target item.
That’s why anti-foaming agents are built into many professional-grade ultrasonic solutions. They ensure that the cleaning bubbles remain microscopic and deep-penetrating, rather than turning the tank into a bubble bath.
Impurities in DIY Mixes Reduce Cleaning Power
Homemade solutions, tap water, or old cleaning liquids often contain minerals, soap scum, or leftover contaminants. These suspended particles not only cloud the solution but also disrupt the ultrasonic energy pathways, leading to uneven cleaning.
Even small levels of impurities can absorb or scatter ultrasonic energy, particularly in high-precision applications like cleaning semiconductor parts or microtools.
If you’re looking for consistent, thorough cleaning—especially across batches—fresh solution and purified water are non-negotiable.
Environmental and Disposal Considerations
As with any cleaning agent, ultrasonic cleaner solutions must be handled responsibly. While many are biodegradable or labeled “green,” that doesn’t mean they can simply be poured down the drain—especially after they’ve been used to remove toxic residues.
Used Solution Is Contaminated Wastewater
Once an ultrasonic solution has been used—particularly in industrial or medical settings—it’s no longer just soap and water. It’s now a slurry of grease, metal particles, oils, biological matter, or even hazardous chemicals.
Pouring this down a regular sink drain can:
- Violate local wastewater regulations
- Damage plumbing over time
- Harm septic systems or aquatic environments
- Create liability in professional workshops or labs
Instead, used ultrasonic solution should be collected and disposed of according to local hazardous waste guidelines. Many facilities require it to be stored in marked containers and picked up by certified disposal services.
Look for Biodegradable, Low-Toxicity Formulas
Many manufacturers now offer eco-friendly ultrasonic cleaning solutions designed for safer use and easier disposal. These typically:
- Avoid phosphates and nonylphenols
- Use plant-derived surfactants
- Break down naturally over time
- Have low aquatic toxicity
However, even biodegradable solutions can become hazardous after use—especially if they remove lead, oil, or biological waste. So always assess the nature of the residue, not just the original solution.
Neutralizing Spent Solutions Before Disposal
Some mildly alkaline or acidic ultrasonic solutions can be neutralized before disposal—but only with proper understanding of chemical handling. For example:
- Acidic solutions (citric or phosphoric acid) may be neutralized with baking soda
- Alkaline solutions (sodium carbonate-based) may be neutralized with vinegar
This process should always be done with protective gear, ventilation, and in a non-reactive container. Even then, check with local authorities before disposal. In commercial or lab environments, neutralization should be handled by trained staff or third-party disposal services.
Reusability and Solution Life Span
A common question is: How often should I change ultrasonic cleaner solution?
It depends on:
- How dirty the items are
- What type of grime is being removed
- Whether the solution is filtered or circulated
In general:
- Light use: Replace every 5–10 cleaning cycles
- Moderate use: Replace daily
- Heavy/industrial use: Replace between batches, or every 4–6 hours
Used solutions should never be “topped up” without draining and rinsing the tank. Otherwise, you’re diluting effectiveness and spreading contamination.
Real-World Examples of Ultrasonic Cleaning Solutions in Action
The versatility of ultrasonic cleaning solutions is best appreciated through real-world use cases. From medical clinics to auto shops and electronics labs, each environment presents different cleaning challenges—and relies on specially formulated solutions to meet them.
Jewelry Store: Restoring Shine Without Damage
A high-end jewelry boutique uses a neutral pH ultrasonic cleaning solution designed for gold, platinum, diamonds, and delicate stones. Their solution contains:
- Mild surfactants to lift oils and residue
- Brighteners to restore luster
- Anti-tarnish agents to preserve metal finishes
The store services dozens of rings, chains, and earrings daily. Using a high-frequency ultrasonic bath with this formula, they achieve consistent shine without abrasive polishing or chemical tarnish removers. Because the solution is gentle, it protects settings, prongs, and coatings.
Dental Clinic: Pre-Sterilization of Instruments
In a busy dental practice, staff use a dual-enzyme ultrasonic solution to clean handpieces, scalers, and curettes before autoclaving. The solution includes:
- Protease and lipase enzymes to break down blood and tissue
- Non-foaming surfactants
- Neutral pH to prevent corrosion
Using warm water (around 40°C), the clinic runs ultrasonic cycles for 10 minutes, then rinses and autoclaves the instruments. The enzymatic solution not only reduces manual scrubbing but also improves sterilization outcomes by removing microscopic organic matter.
Gun Shop: Heavy-Duty Degreasing with Alkaline Cleaner
A firearm maintenance shop specializes in deep-cleaning used rifles and pistols. Their ultrasonic cleaner is paired with a high-alkaline solution containing:
- Emulsifiers for oils and carbon deposits
- Rust inhibitors
- Buffered agents to protect bluing and finishes
This solution breaks down years of residue inside triggers, barrels, and bolts—without requiring disassembly beyond field-stripping. The final rinse leaves no residue, and the rust inhibitors extend protection during air drying.
Electronics Lab: PCB Cleaning with Specialty Formula
An electronics repair facility uses a proprietary ultrasonic solution that’s:
- Alcohol-free
- Anti-static
- Non-conductive
- Residue-free
Used for circuit boards, solder flux removal, and delicate sensor parts, this solution is safe for conformal coatings, fine wires, and adhesives. The cleaner operates at low heat with a high-frequency setting (typically 80–120kHz), and parts are dried using filtered compressed air. No visible film or streaking is left behind, even under microscope inspection.
Manufacturing Plant: Rust Removal from Steel Molds
In an industrial setting, a plant responsible for manufacturing injection molds uses an acidic ultrasonic solution with descaling agents. These include:
- Phosphoric acid
- Chelators
- Wetting agents
Rust and oxide layers are removed within 10–15 minutes. However, operators monitor timing closely and follow with an alkaline rinse and drying process. This preserves surface tolerances and prevents flash rusting after cleaning.
Final Thoughts on Choosing and Using Ultrasonic Cleaning Solutions
Whether you’re cleaning jewelry at home or prepping lab instruments in a sterile facility, the ultrasonic cleaning solution you use is just as important as the machine itself. The best ultrasonic cleaners amplify cleaning power—but only when matched with a compatible, purpose-built formula.
Effective solutions do more than remove grime. They:
- Enhance cavitation
- Protect sensitive materials
- Improve hygiene and safety
- Extend equipment life
- Reduce manual labor and rework
Understanding what’s in your ultrasonic cleaner solution—and how to use it properly—transforms a good cleaning tool into a precise, professional-grade system.
It all starts with choosing the right chemistry for your task.

Granbo Ultrasonic Cleaner
 Granbo Ultrasonic
Granbo Ultrasonic